- Home
- Parkinson’s
- CKBR-12
- Phone Consultation
- Research
- New York Academy Of Sciences
- Genesis Parkinson’s Institute Research
- Treatment of Parkinson’s disease with trophic factors
- Tropane-Based Ibogaine Analog Rescues Folding-Deficient Serotonin and Dopamine Transporters
- Google co-founder backs biotech studying psychedelic African shrub
- Ibogaine modifies GDNF BDNF and NGF
- About Us
- Contact Us
- DONATE
- Home
- Parkinson’s
- CKBR-12
- Phone Consultation
- Research
- New York Academy Of Sciences
- Genesis Parkinson’s Institute Research
- Treatment of Parkinson’s disease with trophic factors
- Tropane-Based Ibogaine Analog Rescues Folding-Deficient Serotonin and Dopamine Transporters
- Google co-founder backs biotech studying psychedelic African shrub
- Ibogaine modifies GDNF BDNF and NGF
- About Us
- Contact Us
- DONATE
© 2026 Genesis Parkinsons Institute
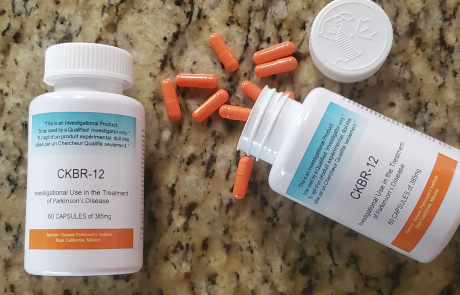
CKBR-12
CKBR-12® (12-methoxyibogamine monohydrochloride) Experimental Use in the Treatment of Parkinson's disease (PD)
CKBR-12® is an Ibogaine Derivative Natural Health Product (NHP) currently under evaluation as an anti-Parkinson medication. Developed by Genesis Parkinson’s Institute SA, a division of Genesis Research, Inc., CKBR-12® is manufactured under GMP conditions in a GMP compliant facility.
CKBR-12® was employed in an experimental protocol in clinical studies in the treatment of non-motor and motor dysfunction symptoms of Parkinson disease (PD).
The trial, which encompassed a holistic patient-driven view of care, demonstrated a rapid amelioration or total elimination of both motor and non-motor symptoms of PD, leading to an overall improvement of patient health-related quality of life (HRQOL).
These unexpected, promising and previously unreported results gave rise to the filing of a US and PCT provisional patent, Rapid Method for Reducing Motor and Non-Motor Symptoms of Parkinson’s disease.
Preliminary results showed an amelioration or total elimination of both non-motor symptoms and motor symptoms within two (2) weeks of the onset of the CKBR-12® treatment.
This has continued in patients monitored for eight years to date.
A tremendous reduced level of bradykinesia and rigidity was reported, which expressed itself in an improved postural stability gait, balance and speech.
No excess saliva and drooling was also noted.
The absence of depression along with anxiety, all cognitive functions and other psychological issues was one of the most promising findings during this 30 day treatment with CKBR-12®
No symptoms have returned
Data was captured using Patient-Reported Outcome (PRO) tools and video to evaluate HRQOL
These observations provide support for the hypothesis that CKBR-12® represents a means of ameliorating and or eliminating non-motor and motor PD symptoms and improving the overall HRQOL of Parkinson patients.
The unexpected findings of CKBR-12® on the severity and occurrence of multiple NMS require FDA approval, as these observed effects could decrease the growing social disability of PD patients significantly and consequently ease the burden on the socio-economic costs for healthcare..
MANUFACTURER’S STATEMENT
CKBR-12® is an investigational product currently under evaluation as an anti-Parkinsonian agent for the treatment of motor and non-motor symptoms of Parkinson’s disease (PD). This product is intended for use by Genesis Parkinson’s Institute only.
The investigational drug is manufactured in a GMP compliant facility under GMP conditions.
The active pharmaceutical ingredient (API) of CKBR-12® is IBOGEN® (Ibogaine HCl).
CKBR-12® contains no animal products, soy, wheat, salt, sugar, milk, yeast, gluten, artificial flavors or preservatives.
Important Disclaimer:
CKBR-12® contains Ibogaine and is strictly intended for use or consumption within Mexico or any countries where it is legal. It must not be transported or carried into any country where Ibogaine is considered illegal or classified as a controlled substance. Failure to comply with local laws could result in legal consequences. Please ensure responsible usage and adherence to all applicable laws and regulations.
CKBR-12® Is not available for sale or use in the United States Of America.
© 2026 Genesis Parkinsons Institute